The new Biomerics office will be equipped with multiple balloon-forming machines, state-of-the-art test equipment, and a cleanroom that is ISO 13485:2016 certified.


The new Biomerics office will be equipped with multiple balloon-forming machines, state-of-the-art test equipment, and a cleanroom that is ISO 13485:2016 certified.
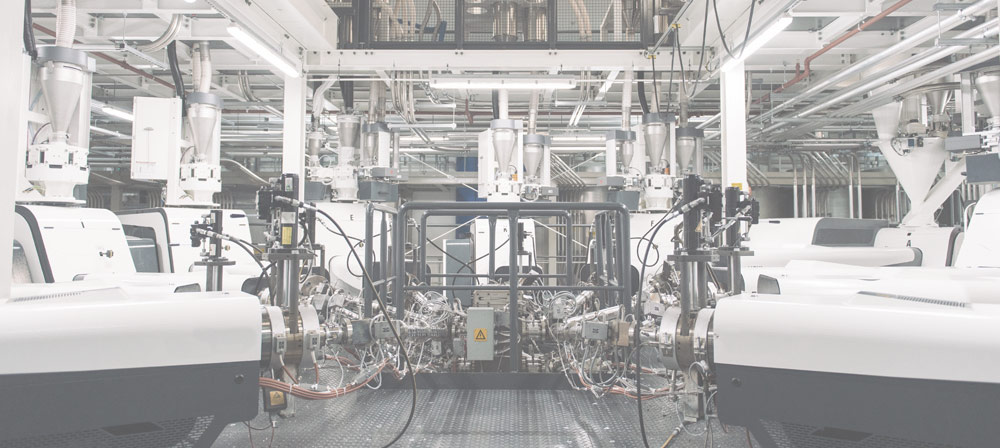
Through this investment, the Sligo site will feature Class 7 cleanroom manufacturing environments and state-of-the art thermoforming operations, fully certified to ISO 13485 standards and meeting the highest regulatory requirements.

Celerion, a clinical research organization (CRO) to the biopharmaceutical industry, has announced completion of additions to its research capacity and services
Novo Holdings today announced that it has co-led with Vivo Capital the over-subscribed US$200 million Series A and Crossover round in Esco Lifesciences Group (“Esco”), a leading provider of life sciences tools and services. The financing will enable Esco to strengthen its position through organic and external growth and transformation, as well as invest in China, thus addressing some of the most dynamic market segments and geographies. Other investors include two Asian sovereign wealth funds and Singapore-based global investor, EDBI.
Novatek Requalified as an approved supplier for Boehringer Ingelheim, a global leader in healthcare. Novatek regulatory compliant quality solutions is used at Boehringer Ingelheim for its human pharma, animal health and biopharmaceutical aseptic (sterile) manufacturing sites worldwide.
Freudenberg Medical, a global contract design and manufacturing provider to the medical device industry, is expanding its manufacturing operations in Alajuela, Costa Rica. The expansion project adds 8,600 square feet to the existing facility which includes construction of an additional ISO Class 7 cleanroom for catheter manufacturing and assembly, molding, extrusion, and packaging, as well as added office space. New technology includes an advanced thermoplastic extrusion line which can produce tubing from 4 inches to 170 inches long with capacity of up to 1 million parts per month, plus additional injection molding machines.
Prenetics Limited, a global leader in diagnostics and genetic testing, Oxford University and Oxford Suzhou Centre for Advanced Research (OSCAR) have signed multi-million dollar collaboration agreements to further develop the award-winning OxLAMP™ technology, a rapid, molecular testing technology for infectious diseases.
soloPURE™ provides a closed, Grade-A, aseptic environment for fill finish activities requiring positive and negative pressures, giving organizations with facility restraints or expensive closed-barrier systems a better way to handle sterile potent powders, including products previously thought to be “out of reach” in conventional barrier systems.
Avantor® to Acquire Ritter GmbH and its Affiliates; Expands Proprietary Offering for Diagnostic and Drug Discovery Workflows, Combining Ritter’s liquid handling consumables platform for clinical diagnostic testing and life sciences research with Avantor’s offerings for critical lab automation workflows and leading global customer channel
Viant announced today that it has completed a major expansion of its medical device manufacturing facility in Heredia, Costa Rica. The company invested in the additional capacity to meet market demand for minimally invasive surgical devices, including energy-based devices. The expansion will benefit customers by enhancing Viant’s ability to provide speed to market for high-quality, complex medical devices.
Matica Biotechnology, Inc. (Matica Bio), a contract development and manufacturing organization (CDMO) specializing in the clinical and commercial production of cell and gene therapies, ceremonially broke ground today on its new 25,000 ft2 facility which will house its GMP virus production suites, development laboratories and company offices. The new building will be located in Providence Park at 2501 Earl Rudder Freeway in College Station.
Cleanroom SCARA Robots to Automate Medical Syringe Manufacturing, Including a COVID-19 Medical Application
NuTec’s Syringe Coating Machine Includes Four Epson G6 Cleanroom SCARA Robots to Precisely, Efficiently, and Cost-Effectively Automate Syringe Manufacturing